Density in Physics
What is Density
Every object in the universe is made up of matter (atoms and molecules) and takes up some space. The measure of how much matter (stuff) there is within a given volume is what we call density. For example, an iron ball and a plastic ball may be the same size, but the iron ball has a much higher density because it contains more mass or matter in the same amount of space.
Density denoted by the letter (ρ) “rho”, is defined as the material’s mass per unit of volume.
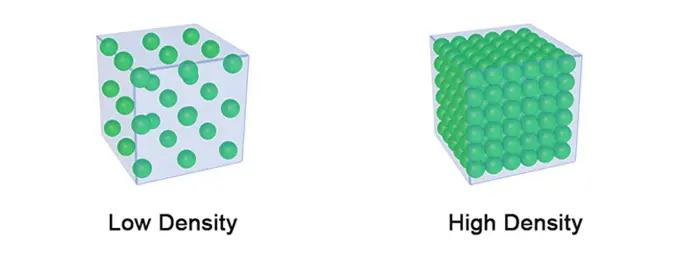
The more matter you can fit per unit of volume, the denser / more compacted a material is. In other words, density tells us how compact or tightly packed matter is within a given volume. The mass and size of atoms, as well as the atomic structure, determine the density of a material.
The density of a material varies with temperature and pressure.
Relative Density
Relative density, also known as specific gravity, is a dimensionless quantity that compares the density of a substance to the density of a reference substance, usually water. It is defined as the ratio of the density of the substance to the density of the reference substance at a specified temperature and pressure.
This concept of describing a material’s density in relation to another has proven to be extremely valuable in engineering, physics, and other fields, especially when working with fluids.
For instance, if we measured the relative density of some arbitrary object with water as our reference material. We will be able to find out whether the material would sink, float, or remain stationary when placed in water. If the relative density turns out to be larger than one, it means the object will sink; if it’s less than one, the object will float; and if the relative density is equal to one, it will remain at the same depth but fully submerged in water.
When the density of one material is greater than that of another, it means that the first material is more compact and has more mass per unit of volume relative to the other material.
Formula & Units
where:
- ρ is the density
- m is the mass of the material
- V is the volume of the material
The SI unit of density is kg/m3. However, it’s common to use g/cm3 for solids, g/ml for liquids, and g/L for gases.
The US customary unit of density is lb/ft3.
Relative density is a unitless value, as it’s the ratio between two similar quantities.
Density Example
Let’s say we want to find the density of a wooden ball with a mass of 150 grams and a volume of 230 cm3.
To find the density, all we need to do is divide the mass (150 g) by the volume (230 cm3)
So the density of the wooden ball would be:
150 g / 230 cm3 ≈ 0.65 g/cm3.Relative Density Example
Suppose we want to find out if an apple would float on water. We did some research and found out that the density of an apple is approximately 0.75 g/cm3, and for water, it’s 1 g/cm3.
To find out if an apple would float on water, we just need to divide the density of the apple 0.75 g/cm3 by that of water 1 g/cm3:
0.75 g/cm3 / 1 g/cm3 = 0.75And since it’s less than one, it means that an apple would float when placed in water.
Factors Affecting Density
There are several factors that can affect the density of a material or object. Here are some of the most important ones:
1) TemperatureGenerally, an increase in temperature causes a decrease in density for most materials. When the temperature of a material increases, its volume tends to expand (due to a physical property known as thermal expansion); therefore, the matter within the material would have more space between them, resulting in a decrease in the material’s density.
However, there are some materials for which the opposite is true. For example, water has a unique property where its density actually increases as it is heated from 0°C to 4°C, before decreasing again at higher temperatures. This is due to the structure of water molecules and the way in which they form hydrogen bonds at different temperatures.
2) PressureThe density of a material is directly proportional to pressure. That is, an increase in pressure leads to an increase in density and vice versa. When we increase the pressure applied to a material, it gets compressed, reducing its volume and causing the matter within that material to get more squeezed together. This results in an increase in the mass per unit volume of the material, thus increasing its density.
This effect is particularly noticeable in gases, which are highly compressible and therefore have a very strong pressure-density relationship. For example, at standard temperature and pressure (STP), the density of air is approximately 1.2 kg/m3. However, if the pressure on the air is increased by a factor of 10, the density will increase to approximately 12 kg/m3.
3) CompositionThe composition of a material can have a significant effect on its density. Different materials have different atomic and molecular structures, which can affect how closely packed their particles are and how much space they occupy. For example, materials with a high atomic weight, such as metals like lead or gold, tend to have higher densities than lighter materials like wood or plastics.
In addition to the type of atoms or molecules in a material, the arrangement of these particles can also impact density. For instance, a material with a tightly-packed, crystalline structure will generally have a higher density than a material with a looser, amorphous structure.
4) PorosityThe porosity of a material refers to the amount of empty space or pores within it. Materials with high porosity will have a lower density because there is less mass per unit volume. For example, a sponge has a lower density than a solid piece of rubber because it contains a lot of empty space.
5) State of matterThe state of matter can also affect density. In general, the density of a material is highest in its solid state, intermediate in its liquid state, and lowest in its gaseous state.
This is because the particles in a solid are tightly packed and have little room to move around, resulting in a high density. In contrast, the particles in a liquid are more loosely packed and can move around more freely, resulting in a lower density than in a solid. The particles in a gas are even further apart and can move freely in any direction, resulting in the lowest density of the three states.
However, there are exceptions to this general trend. For example, ice, which is the solid state of water, has a lower density than liquid water due to the unique crystal structure of ice that causes it to occupy more space than liquid water.
Applications of Density
Density is a fundamental concept in physics, and it has many practical applications in various fields, including materials science, engineering, and geology. Here are some examples of how density is used in physics:
- Engineering: Density is used in engineering to design and analyze structures and components. For example, engineers use density to calculate the weight of a component and ensure that it can support the loads it will encounter during its service life. Density is also used to determine the buoyancy of objects and calculate the volume of fluids in hydraulic and pneumatic systems.
- Geology: Density is also used in geology to study the composition and structure of the Earth’s crust. By measuring the density of rocks and minerals, geologists can infer their composition and identify different types of rock formations. Density is also used to study the subsurface structure of the Earth, such as in the exploration of oil and gas reserves.
- Fluid Mechanics: Density is a fundamental property in fluid mechanics that plays a critical role in characterizing and analyzing the behavior of liquids and gases. It is used to determine various properties of fluids, including their pressure, velocity, and flow rate as they move through pipes, channels, and other conduits. Moreover, density is essential in studying fluid dynamics, which is crucial in designing aircraft wings and propellers, among other things.
Density Summary | ||
|---|---|---|
| Definition | The mass of a substance per unit volume | |
| Symbol | ρ or D | |
| Formula | ||
| Units | Si unit (Kg/m3) | US unit (lb/ft3) |
Frequently Asked Questions
- What is mass?
- Mass is a measure of the amount of matter in an object or substance.
- What is volume?
- Volume is the space occupied by an object. For example, a cube with a side length of 1 cm would occupy 1 cm in length, width, and height, thus a volume of 1 cm3.
- What is the difference between mass and weight?
- Mass is an intrinsic property of a material that measures the amount of matter in an object. Weight, on the other hand, is simply the force product of mass times the acceleration due to gravity. For instance, the weight of an object on earth would be different than its weight on the moon since gravity is different, but its mass would be the same.
- What is the densest element?
- Osmium is the densest known element.
- What is the least dense element?
- Hydrogen is the least dense known element.
- What is bulk density?
- Bulk density is the mass of the material divided by the total volume it occupies. The total volume includes both the solid particles and the pore volume.
- Can density be negative?
- Density is the measure of mass per unit volume, and since neither mass nor volume can be negative, therefore density can never be negative.
- What density floats in water?
- Any material or object that has a density less than the density of water, which is (997 kg/m3) at room temperature, will float when placed in water.
